-
-
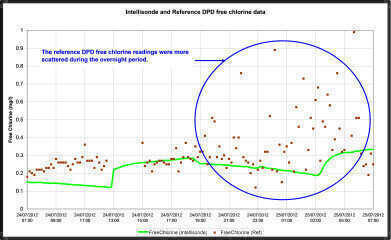 Figure 1
Figure 1 -
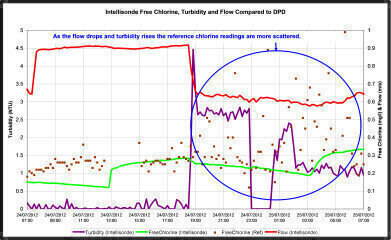 Figure 2
Figure 2
Water/Wastewater
Chlorine mystery solved
Apr 10 2013
Multiparameter Intellisondes have enabled water supply engineers to identify the source of a problem which had been creating highly variable chlorine readings in desalinated water.
Following the installation of in-pipe drinking water quality monitoring sondes, distribution engineers discovered an occasional significant disparity between the Intellisondes’ data and results from grab samples tested by a photometer tablet (DPD) method. For most of the time, there was close agreement between the two methods, but periodically, the photometer results varied wildly, whilst the in-pipe monitor showed relatively stable values.
Initially, distribution managers suspected that the online monitors were at fault and that they were failing to detect occasional spikes in free chlorine. However, as a multiparameter monitor, the Intellisonde was able to provide more clues which ultimately lead to the successful identification of the problem.
Background
Intellisondes™ provide real-time monitoring data for water within pipeline distribution networks. They feature up to 12 sensors inside a tiny sonde head that fits in a water pipe through a 3.8 or 5cm valve. Measurement options include Free Chlorine, Mono-chloramine, Dissolved Oxygen, Conductivity, pH, ORP/Redox, Flow, Pressure, Temperature, Turbidity and Colour. An ISE channel is also available for Fluoride, Ammonium and Nitrate.
The water supply in this study was from a desalination plant. Infrastructure is fairly new and the water company takes water from a processor and a trunk distributor. The Intellisondes at these sites monitored free chlorine, temperature, pH, conductivity, turbidity and flow, at fifteen minute sampling intervals.
The solid-state free chlorine sensor in the Intellisonde employs electrochemical technology to provide accurate data without the need for reagents or other consumables.
In order to check the performance of the Intellisondes, grab samples were taken and free chlorine tests were conducted with a portable photometer which measured the colour intensity produced when DPD tablets were crushed and mixed with a sample. This test method that has been in common practice for decades.
The problem
In an initial study, regular samples were taken manually over a 24 hour period and tested with the DPD method. These results were compared with the Intellisondes’ logged free chlorine data - see Figure 1.
An unexpected scatter of DPD measurements was observed during night time and on multiple occasions.
The solution
By providing continuous data for other parameters, (in this case, temperature, pH, conductivity, turbidity and flow) the Intellisondes were able to highlight other factors that could be affecting the DPD measurements.
The data in Figure 2 shows that at the time when the DPD measurements were most scattered; flow dropped and turbidity increased, so it was suspected that the particles contributing to this turbidity could be interfering with the DPD measurement. The process of sampling caused flow disturbance in the pipe which appeared to result in a variable level of particles in each sample; low flows at night were resulting in the settlement of particles which were disturbed (randomly) by the sampling process, and it was considered likely that these particles were affecting the DPD results.
In order to check this assumption, local sand was added to bottled drinking water and tested by the DPD method. This water had previously been tested and shown not to contain free chlorine, but following the addition of local sand the DPD test gave a positive result for free chlorine. This confirmed that sand was the cause of the false DPD data.
Subsequently, samples of water taken from the Intellisonde locations were allowed to settle and then showed no chlorine in a DPD test. However, when the samples were shaken, DPD tests erroneously reported the presence of chlorine.
The reagents in DPD tablets are known to react with a wide range of species, including iron and manganese compounds, and it was discovered that local sand grains have a coating of iron oxide which gives them a pink colour. It is highly likely therefore, that this iron oxide coating was reacting with the DPD reagent and causing the false free chlorine measurements.
Expressing her satisfaction with the success of this investigative work, Intellitect Water’s Jo Cooper said: “This project highlights the benefits of multiparameter monitoring - without the flow and turbidity data, we might never have surmised that the DPD results were incorrect and that sand was the cause.”
Digital Edition
IET 34.2 March 2024
April 2024
Gas Detection - Biogas batch fermentation system for laboratory use with automatic gas analysis in real time Water/Wastewater - Upcycling sensors for sustainable nature management - Prist...
View all digital editions
Events
Apr 30 2024 Melbourne, Australia
Apr 30 2024 Birmingham, UK
May 03 2024 Seoul, South Korea
May 05 2024 Seville, Spain
May 06 2024 Minneapolis, MN, USA

















