Environmental Laboratory
Updated reference standards’ packaging, labelling and certificates revamped for improved laboratory safety and productivity
Dec 06 2022
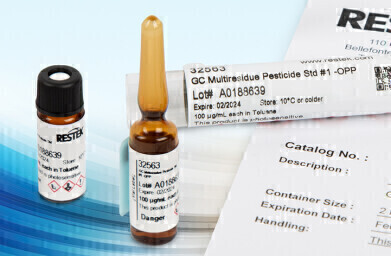
Restek have made some significant improvements to its packaging, labelling and certificates of analysis for their ISO-accredited reference standards. They have made their round ampul storage tubes smaller to ensure that the Restek ampuls, including the deactivated vial, fit perfectly to avoid breakages, safety hazards and delays. These tubes take up less space; three of the new designed tubes use up the same amount of space as one of the old. As they use less plastic, these new tubes are also kinder to the environment.
In addition, the ampul storage tube label now has a QR code with a direct link to the certificate of analysis for the operator’s specific standard and lot #. If you want to archive your certificate digitally or cannot find the hard copy, the QR code provides instant, easy access to the necessary documentation whenever or wherever needed. Moreover, larger pictograms on the Globally Harmonized System (GHS) compliant labels improve laboratory safety while helping to comply with OSHA, CLP, and other international legislative criteria.
Restek’s ampul labels are now removable so they can be transferred to the included deactivated vial along with the standard. The vial and notebook labels remain on the ampul until they are ready for use. The new and larger notebook includes “received” and “opened” write-in date areas. Restek’s new vial label is the ideal size to provide clear visibility to the contained volume and trouble-free autosampler integration without trimming. Both labels also include complete handling instructions to assist end-users to achieve integrity and proper use of their standards before and after transfer.
Restek collaborated with its independent ISO accreditation body representatives to streamline and simplify documentation, avoid confusion and enable end-users to report with confidence. Specifically, Restek certificates now provide a single “total expanded uncertainty” value rather than the three uncertainty values provided historically. Restek have also carried out extensive, real-time travel studies to improve the precision of the now-single uncertainty value. In particular for Custom Restek reference standards now have their three levels of available documentation retitled; each is clearly and accurately marked as a different type of “certificate of analysis.” Each level’s definitions have been updated to define the differences.
Restek has not changing their manufacturing processes, QC methods, ISO accreditations, CRM designations, ampuls, or the composition of the standards at all. Customers can continue to order the same products/catalogue numbers and Restek will provide receive the same high-quality standards they always have.
Digital Edition
AET 28.2 April/May 2024
May 2024
Business News - Teledyne Marine expands with the acquisition of Valeport - Signal partners with gas analysis experts in Korea Air Monitoring - Continuous Fine Particulate Emission Monitor...
View all digital editions
Events
Jul 30 2024 Jakarta, Indonesia
China Energy Summit & Exhibition
Jul 31 2024 Beijing, China
2024 Beijing International Coal & Mining Exhibition
Aug 07 2024 Beijing, China
IWA World Water Congress & Exhibition
Aug 11 2024 Toronto, Canada
Aug 25 2024 Stockholm, Sweden and online








.jpg)








