-

-
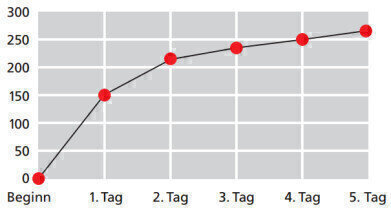 Figure 1: BOD5 curve with a sample volume of 157 ml
Figure 1: BOD5 curve with a sample volume of 157 ml
Water/Wastewater
BOD - The Key Parameter of Water Treatment
Apr 07 2015
As a result of the increasing standards of living over the last few years, water consumption and demand for its availability are growing constantly on a global scale. Efficient water treatment can reduce contamination as well as improve water quality. It also supports the natural self-purification quality of water.
One of the most important parameters to assess water quality based on organic impurities is the Biochemical Oxygen Demand (BOD). The BOD value has played an important role in the treatment and purification of wastewater since the 19th century. The effectiveness as well as the handling of these methods have been continually advanced ever since. The BOD value in combination with COD (Chemical Oxygen Demand) and TOC (Total Organic Carbon) is widely used as an indication of the contamination of water samples. BOD is also indispensable for ensuring the efficient and cost-effective water treatment in modern wastewater treatment plants. A distinction is made between different measurement methods used to determine the BOD value. A standard method is the respirometric method adapted from DIN 38 409-H52 / EN 1899-2. The BOD5 value is widely known where the demand of oxygen is measured over five days in order to produce reliable and reproducible measurement results. Some countries perform this analysis over longer periods for example a BOD7 value.
This measurement is performed in an incubated system. The microorganisms contained in wastewater samples consume oxygen and produce carbon dioxide. Potassium hydroxide (KOH) that absorbs this carbon dioxide is added in the container above the sample. From the drop of pressure, the sensor electronics computes a value that is in direct proportion to the BOD value. To achieve reproducible results, the measurement is performed at a constant temperature of 20 °C.
The measurement result is specified in mg oxygen / l water sample. Figure 1 shows the oxygen demand of a typical water sample in relation to the measurement period. Based on this plotted line, it can be established after a relatively short period of time whether the measured results are within the tolerance range and if the measurement is correct. This flexibility allows wastewater treatment processes to be quickly adapted to the contamination level of the water. For this reason, it is particularly beneficial to use a BOD appliance with a visual graphics display of the measurement results.
The measurement of the BOD value is influenced by the temperature, pH value and depends on a sufficient amount of oxygen and nutrient content, the C-N-P ratio. It is also sometimes advisable to introduce added nutrients (e.g. ammonium chloride).
Leakages in the measurement system lead to inaccurate and non-reproducible results. Nitrification may also cause significant alterations in the results. This involves the oxidisation process as part of the degradation of nitrogen with nitrifying bacteria. Then, the measured drop in pressure is not just attributed to the consumption of oxygen that is required for the degradation of organic carbon compounds. Higher values are measured that do not correspond to the actual BOD values. This unwanted side reaction can be suppressed by adding a nitrification inhibitor such as allylthiourea (ATH) in order to produce a sufficiently accurate BOD value.
Digital Edition
AET 28.2 April/May 2024
May 2024
Business News - Teledyne Marine expands with the acquisition of Valeport - Signal partners with gas analysis experts in Korea Air Monitoring - Continuous Fine Particulate Emission Monitor...
View all digital editions
Events
Jul 30 2024 Jakarta, Indonesia
China Energy Summit & Exhibition
Jul 31 2024 Beijing, China
2024 Beijing International Coal & Mining Exhibition
Aug 07 2024 Beijing, China
IWA World Water Congress & Exhibition
Aug 11 2024 Toronto, Canada
Aug 25 2024 Stockholm, Sweden and online








.jpg)








